Introduction: PD-1 inhibits the cytotoxic T-cell functions via the interaction with its ligands (PDL1 and PDL2). Recently, our group showed a selective response (~40%) with PD-1 blocking antibody pembrolizumab in patients with Richter transformation (RT), particularly after prior exposure to ibrutinib (Wei Ding et al, Blood, 2017). Additionally, PD-1 showed an increased expression in tumor B-cells of patients with RT (Rong He et al, AJSP, 2018). Here we investigated the functional implications of the PD-1 signaling axis in lymphoma B-cell pathobiology.
Methods: 26 CLL-involved lymph node (LN) and 20 RT-involved LN samples were tested for PD-1 expression by immunohistochemistry (mouse clone NAT105, Abcam). Then, we checked PD-1 expression in 10 lymphoma cell lines and 1 CLL cell line (MEC-1) by both, flow cytometry and Western blot (WB) analysis. Over-expression of PD-1 using pLEX-lentiviral system (Thermo Scientific) was evaluated for in vitro cell cycle regulation and in vivo tumor growth. Overexpression of PD-1 was further examined on the regulation of cell signaling pathways using human phospho kinase array kit (R&D) and WB analysis. Gene expression signatures in CLL and RT patients were also identified by Illumina-based RNA sequencing using Formalin-Fixed Paraffin-embedded (FFPE)-nodal tissue obtained by clinical biopsy (Tempus Labs; Chicago, IL).
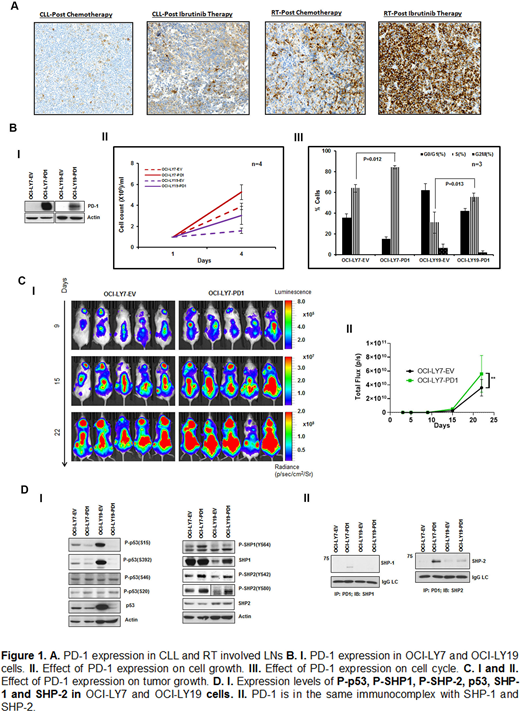
Results: The expression of PD-1 was significantly increased in RT-LN compared to CLL-LN (mean ± SEM in RT vs. CLL, 30.6% ± 4.7% vs. 11.5% ± 2.8%, p < 0.001) [Fig 1A]. PD-1 expression was highest in patients with RT where the last prior CLL therapy was ibrutinib. To test the role of PD-1 in lymphoma B-cells, its expression was assessed in lymphoma and CLL cell lines. The expression of PD-1 was found to be variable, but at very low-levels in lymphoma lines OCI-LY7 and OCI-LY19 (data not shown). Constitutive lentiviral (pLEX-PD-1)-mediated overexpression of PD-1 in OCI-LY7 and OCI-LY19 cells led to increased cell growth (1.4 and 1.9 fold compared to original lines on day 4, respectively, Fig 1BI-II), which was further confirmed by cell cycle analysis which showed an increase in S phase by 20.4% and 24.53% in OCI-LY7 and OCI-LY19 cells (Fig 1B III), respectively. When the luciferase + PD-1 expressing OCI-LY7 cells were injected intravenously (1X106 cells) into NSG mice, increased tumor growth was observed at 22 days of follow up by bioluminescence imaging (p<0.01, Fig 1C). Using phospho-kinase array, an overall decrease of phosphorylation was detected on multiple sites of p53 (S392, S15, S46) and CHK2 (T68) (data not shown). This finding was further confirmed by WB analyses (Fig1DI). In addition, decreased of total p53 and increased phosphorylation on both SHP-1 and SHP-2 were found in PD-1 overexpressing OCI-LY19 and OCI-LY17 cell lines (Fig 1DI). Immunoprecipitation experiments with anti-PD1 antibody showed that PD-1 was in the same complex with SHP-1 and SHP-2 in OCI-LY7 cells but only with SHP-2 in OCI-LY19 cells (Fig 1DII). In parallel, mRNA sequencing was performed on 11 nodal tissues from either RT (n=8) or progressive CLL (n=3) after they developed clinical progression. The expression of PD-1 (PDCD1) was positively correlated with the expression of SHP-1 (PTPN6, r=0.56) and Cyclin E1 (CCNE1, r=0.62), implicating a direct role of PD-1 in regulating cellular proliferation and the induction in phosphatase expression in RT and CLL.
Conclusion: Our data support the notion that PD-1 overexpression in lymphoma cells modifies cell proliferation in vitro and in vivo and regulates the function of phosphatase SHP and p53- pathways. These findings also indicate that aggressive lymphoma or CLL leukemic cells have the ability to hijack the PD-1 pathway and may result in downregulation of the p53 mediated DNA repair. This novel information provide for new strategies to further evaluate the interaction of checkpoint signals with DNA repair pathway, and possibly provide novel targets for Richter's transformation.
Ding:alexion: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Astra Zeneca: Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; DTRM: Research Funding; MEI Pharma: Membership on an entity's Board of Directors or advisory committees. Sakemura:Humanigen: Patents & Royalties. Parikh:Janssen: Honoraria, Research Funding; Merck: Research Funding; Pharmacyclics: Honoraria, Research Funding; Ascentage Pharma: Research Funding; GlaxoSmithKline: Honoraria; MorphoSys: Research Funding; AstraZeneca: Honoraria, Research Funding; Verastem Oncology: Honoraria; Genentech: Honoraria; AbbVie: Honoraria, Research Funding; TG Therapeutics: Research Funding. Wang:Novartis: Research Funding; Incyte: Research Funding; Innocare: Research Funding. Liu:Eisal: Research Funding; Genentech: Research Funding; GRAIL: Research Funding; Menarini Silicon Biosystems: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Tesaro: Research Funding. Braggio:DASA: Consultancy; Bayer: Other: Stock Owner; Acerta Pharma: Research Funding. Ansell:Seattle Genetics: Research Funding; Takeda: Research Funding; AI Therapeutics: Research Funding; ADC Therapeutics: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Bristol Myers Squibb: Research Funding. Kenderian:Sunesis: Research Funding; Tolero: Research Funding; Torque: Consultancy; BMS: Research Funding; Gilead: Research Funding; Kite: Research Funding; Humanigen: Consultancy, Patents & Royalties, Research Funding; Mettaforge: Patents & Royalties; Novartis: Patents & Royalties, Research Funding; MorphoSys: Research Funding; Lentigen: Research Funding; Juno: Research Funding. Kay:Acerta Pharma: Research Funding; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Juno Theraputics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; MEI Pharma: Research Funding; Sunesis: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharma: Membership on an entity's Board of Directors or advisory committees; Cytomx: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.